APPLY: Superior and substantial Hb increases were achieved with FABHALTA over C5is through the 24-week randomized treatment period
A head-to-head study of C5i-experienced (eculizumab or ravulizumab) adults with PNH and Hb <10 g/dL1
PRIMARY END POINTS
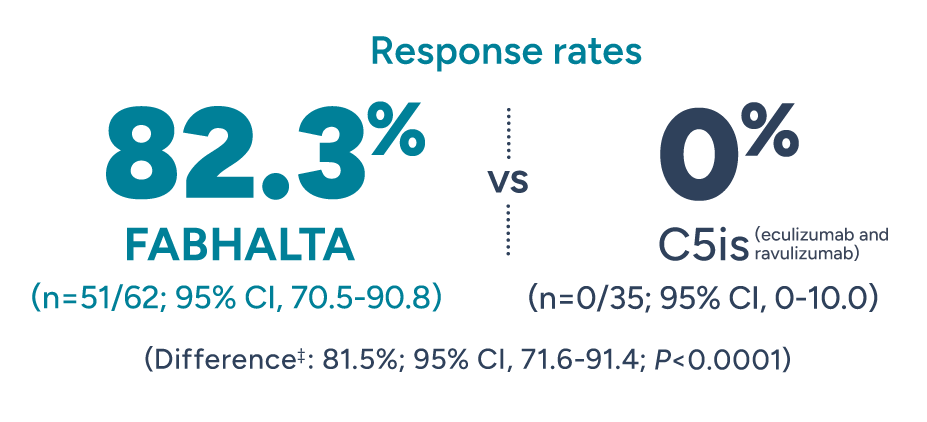
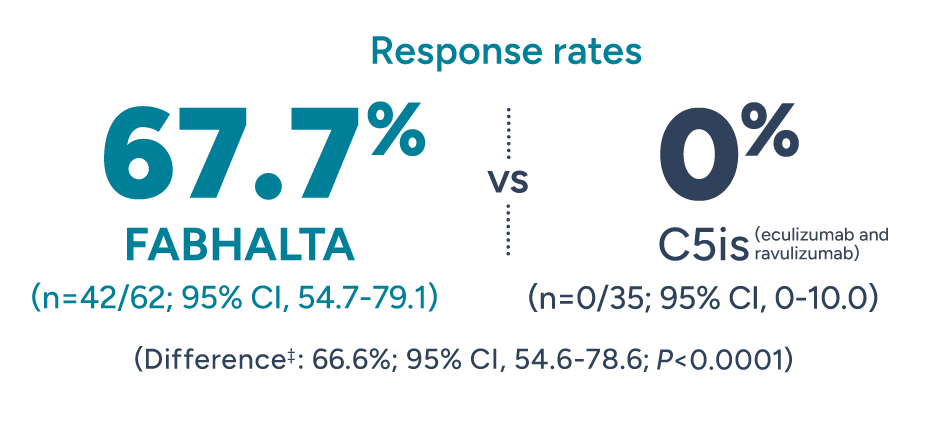
Significantly more patients achieved Hb improvements in the absence of RBC transfusions with FABHALTA vs C5is1
Patients with Hb increase of ≥2 g/dL* from baseline in the absence of RBC transfusions† after 24 weeks
Patients with normalized§ Hb of ≥12 g/dL* in the absence of RBC transfusions† after 24 weeks
*Adjusted mean assessed between Weeks 18 and 24 (Days 126 and 168). Excludes values within 30 days post-transfusion.1
†Assessed between Days 14 and 168. Requiring RBCs refers to any patient receiving transfusions or meeting protocol-defined criteria.2
‡Adjusted difference in proportion.1
§Normalization defined as meeting the primary end point of Hb ≥12 g/dL.2 Normal Hb levels vary but generally are 12-16 g/dL for women and 13-18 g/dL for men.3
Help deliver groundbreaking results without RBC transfusions1
FABHALTA delivered a +3.6 g/dL adjusted mean change in Hb levels from baseline vs -0.1 g/dL with C5is after the 24-week randomized treatment period
ADDITIONAL END POINT
Patients experienced a +1.9 g/dL adjusted mean change in Hb as early as Week 1 with FABHALTA4
Mean Hb levels (g/dL) through Week 24 (values within 30 days of transfusion were excluded and considered missing)4
Click on image to enlarge.
The data in the figure above are exploratory, presented for observation only. No formal conclusions or comparisons between the 2 treatment arms can be made.
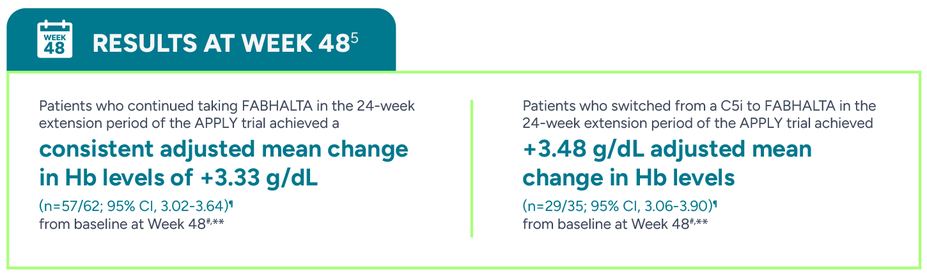
∥Assessed between Days 126 and 168.1
¶The 'n' values reflect patients with non-missing change from baseline.5
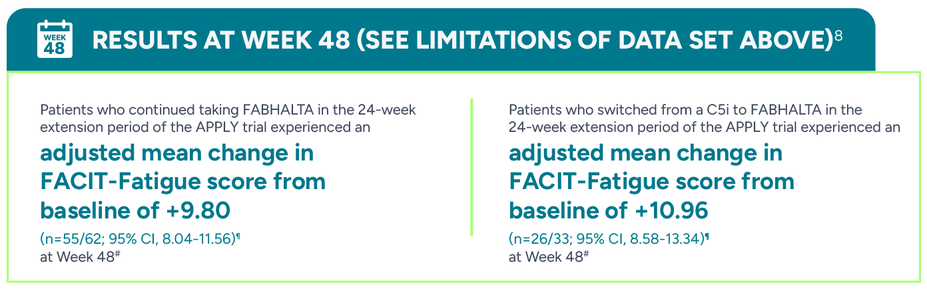
#Adjusted mean assessed at a single Week 48 (Day 336) visit.5
**Hb data during the randomized period and within 30 days post-transfusion were considered missing.5
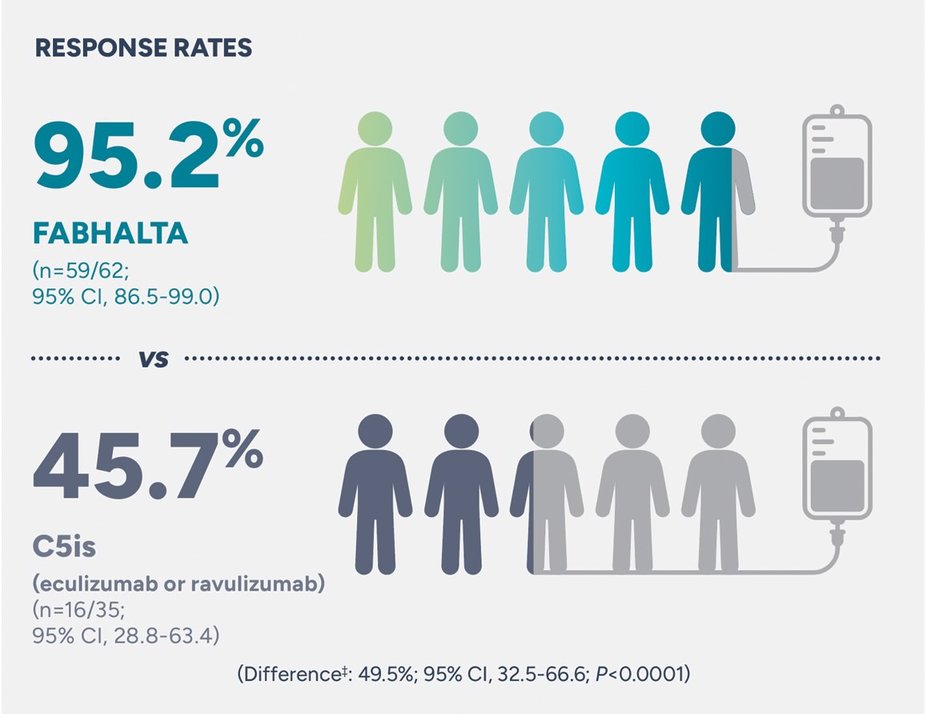
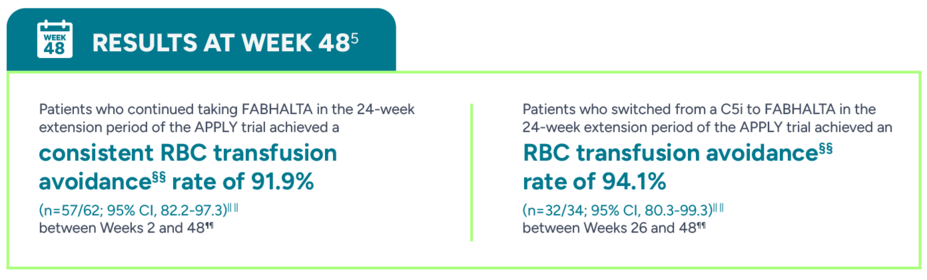
Nearly all patients taking FABHALTA remained free from RBC transfusions through the 24-week randomized treatment period
ADDITIONAL END POINT
RBC transfusion avoidance assessed between Weeks 2 and 24††, ‡‡
‡Adjusted difference in proportion.1
††Assessed between Days 14 and 168.1
‡‡Transfusion avoidance is defined as absence of administration of packed-RBC transfusions between Days 14 and 168.1
35/62 patients in the FABHALTA arm and 21/35 in the C5i arm had at least 1 RBC transfusion in the 6 months prior to trial enrollment2
§§Transfusion avoidance was defined as patients who did not receive RBC transfusion during the specified period.5
||||The 'n' values reflect patients who responded based on observed data.5
¶¶Based on observed data. No multiple imputation framework was used. Analysis considered patients who received transfusions between Days 14 and 336 after the start of FABHALTA treatment and with missing Day 336 assessment as nonresponders.5
What could fewer RBC transfusions mean to your patients?
FABHALTA goes beyond C5is to significantly reduce absolute reticulocyte count (ARC)—a known marker of both EVH and IVH
ARC and lactate dehydrogenase (LDH) are 2 of the known biomarkers of hemolytic activity. ARC is a marker of both IVH and EVH, while LDH primarily reflects IVH.6-8
ADDITIONAL END POINT
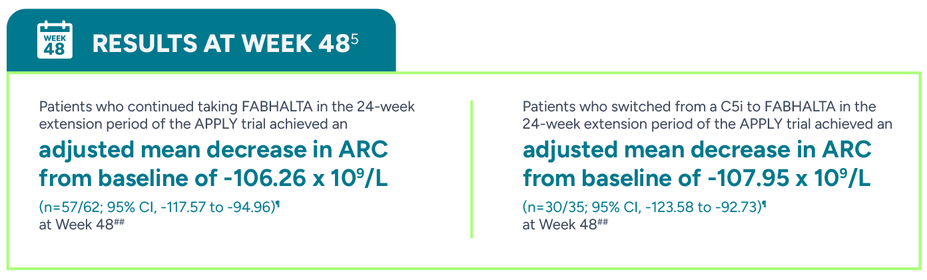
FABHALTA delivered greater reductions in ARC vs C5is1
∥Assessed between Days 126 and 168.1,4
¶The ‘n’ values reflect patients with non-missing change from baseline.5
##Adjusted mean assessed at a single Week 48 (Day 336) visit. Analysis based on observed and central laboratory data only.5
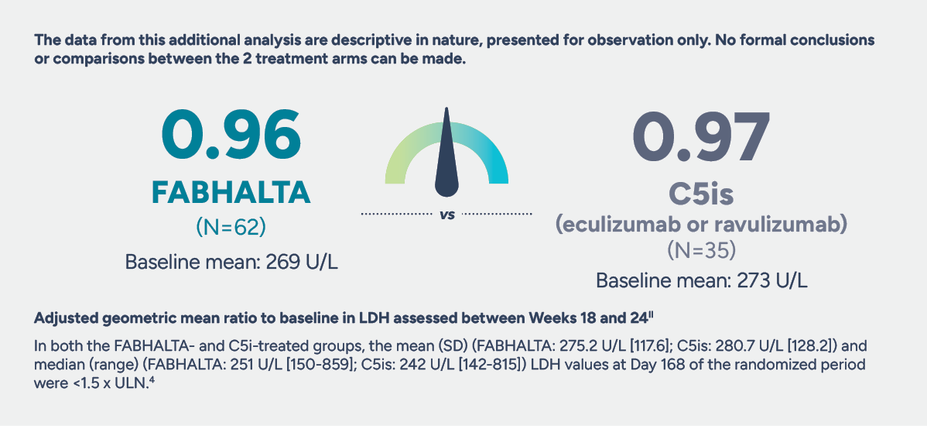
The impact of FABHALTA on LDH levels
ADDITIONAL END POINT
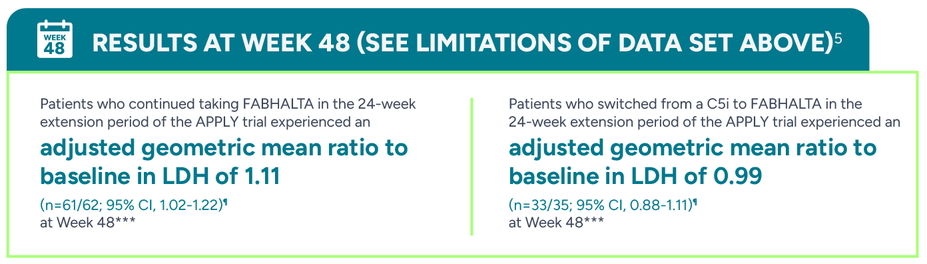
No statistically significant difference in LDH was seen between FABHALTA and C5is in the 24-week randomized treatment period1,9
∥Assessed between Days 126 and 168.1,4
¶The ‘n’ values reflect patients with non-missing change from baseline.5
***Adjusted geometric mean ratio assessed at a single Week 48 (Day 336) visit. Analysis based on observed and central laboratory data only.5
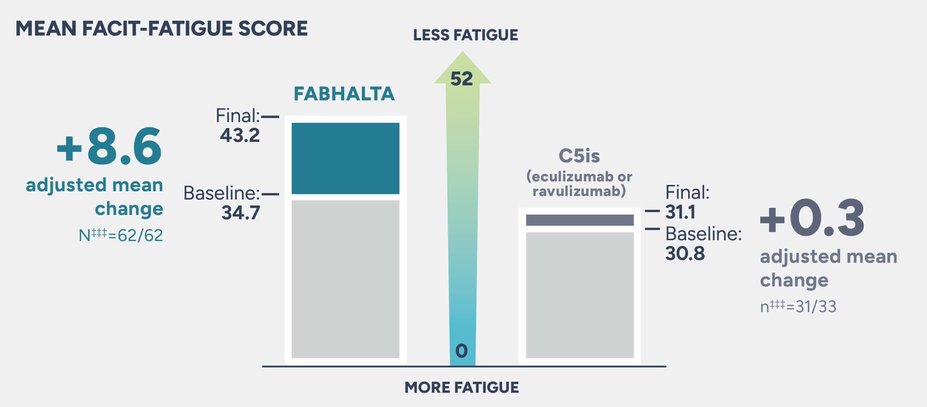
Patients on FABHALTA experienced an adjusted mean change of +8.6 from baseline in FACIT-Fatigue scores vs +0.3 with C5is
ADDITIONAL END POINT
Patient-reported FACIT-Fatigue scores may be an underestimation or overestimation because patients were not blinded to treatment.
The data from this additional analysis are descriptive in nature, presented for observation only. At baseline, ~50% of participants reported the least severe response categories (“not at all” and “a little bit”) for the 10/13 questions on the FACIT-Fatigue scale. Due to the small sample size, open-label design, and the low level of fatigue reported at baseline, no formal conclusions or comparisons between the 2 treatment arms can be made.
During the 24-week randomized period, the adjusted mean change from baseline in FACIT-Fatigue score††† was +8.6 in patients taking FABHALTA and +0.3 with C5is (difference: 8.34; 95% CI, 5.26-11.41)4
The adjusted mean∥ was assessed between Weeks 18 and 24. Values within 30 days after transfusion were included in the analysis4
∥Assessed between Day 126 and 168.2
†††The Functional Assessment of Chronic Illness Therapy–Fatigue Scale (FACIT-Fatigue) is a 13-item questionnaire that assesses self-reported fatigue and its impact upon daily activities and function. The level of fatigue is measured on a 4-point Likert scale (in the study, 4=not at all fatigued to 0=very much fatigued), with 0 being the worst possible score and 52 the best.2
‡‡‡Baseline mean FACIT-Fatigue scores were reported for 62 patients in the FABHALTA arm and 33 patients in the C5i arm.4 At Day 168, the adjusted mean change in FACIT-Fatigue score was reported for 62 patients in the FABHALTA arm and 31 patients in the C5i arm.2
¶The ‘n’ values reflect patients with non-missing change from baseline.5
#Adjusted mean assessed at a single Week 48 (Day 336) visit.5
In separate large-scale surveys, the mean FACIT-Fatigue score for the general population was 4410,11,§§§
§§§The FACIT-Fatigue score for the general population was determined through the assessment of 1010 adults in the US in 2002 and 2426 adults in Germany in 2018.10,11