Safety profile of FABHALTA
Serious infections caused by encapsulated bacteria1
FABHALTA, a complement inhibitor, increases the risk of serious infections, especially those caused by encapsulated bacteria, such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b. Life-threatening and fatal infections with encapsulated bacteria have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.1
Because of the risk of serious infections caused by encapsulated bacteria, FABHALTA is available only through a REMS program called the FABHALTA REMS.1
Please click here to learn more.
Contraindications
FABHALTA is contraindicated:
In patients with serious hypersensitivity to FABHALTA or any of the excipients1
For initiation in patients with unresolved serious infection caused by encapsulated bacteria, including Streptococcus pneumoniae, Neisseria meningitidis, or Haemophilus influenzae type b1
APPLY: A head-to-head study of C5i-experienced (eculizumab or ravulizumab) adults with PNH1
Safety profile of FABHALTA in the head-to-head APPLY trial vs C5is
Adverse reactions reported in >5% of adults with PNH treated with FABHALTA in APPLY (24-week randomized treatment period)
ADVERSE REACTIONS | FABHALTA (N=62) n (%) | C5is (eculizumab or ravulizumab) (N=35) n (%) |
Headachea | 12 (19) | 1 (3) |
Nasopharyngitisb | 10 (16) | 6 (17) |
Diarrhea | 9 (15) | 2 (6) |
Abdominal paina | 9 (15) | 1 (3) |
Bacterial infectionc | 7 (11) | 4 (11) |
Nausea | 6 (10) | 1 (3) |
Viral infectiond | 6 (10) | 11 (31) |
Arthralgia | 5 (8) | 1 (3) |
Thrombocytopeniaa | 4 (6) | 0 |
Dizziness | 4 (6) | 0 |
Systemic hypertensiona | 4 (6) | 0 |
Lipid disordere | 4 (6) | 0 |
aIncludes similar terms.
bNasopharyngitis contains: rhinitis allergic, upper respiratory tract infection, pharyngitis, rhinitis.
cBacterial infection contains: pyelonephritis, urinary tract infection, bronchitis bacterial, bronchitis haemophilus, cholecystitis, folliculitis, cellulitis, arthritis bacterial, sepsis, klebsiella infection, staphylococcal infection, Pseudomonas infection, hordeolum, pneumonia bacterial.
dViral infection contains: COVID-19, herpes zoster, oral herpes, nasal herpes, influenza A virus test positive, influenza.
eLipid disorder contains: dyslipidemia, blood cholesterol increased, low-density lipoprotein increased, hypercholesterolemia, blood triglycerides increased, hyperlipidemia.
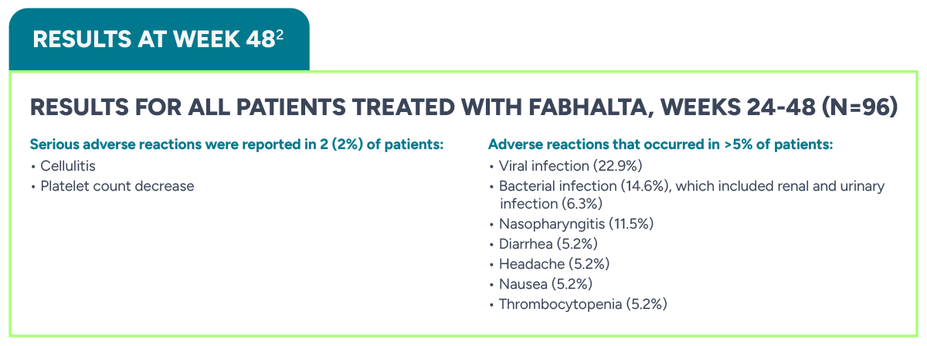
Serious adverse reactions were reported in 2 (3%) patients with PNH who received FABHALTA. They included pyelonephritis, urinary tract infection, and COVID-19
Rash was reported in 2 patients (3%)
Of the 37 FABHALTA-treated patients who had normal platelet counts at baseline, 43% experienced any grade thrombocytopenia during the randomized treatment period
Three FABHALTA-treated patients experienced decreased platelets that worsened to grade ≥3 from baseline (1 patient with normal platelets that worsened to grade 4; 1 patient with baseline grade 1 that worsened to grade 4; and 1 patient with baseline grade 3 that worsened to grade 4)
No patient discontinued FABHALTA or C5is due to an adverse reaction during the 24-week randomized treatment period and no patient discontinued FABHALTA due to an adverse reaction during the 24-week extension phase. Two patients discontinued FABHALTA due to pregnancy (one each in the randomized treatment period and extension period).2,3
Clinical breakthrough hemolysis (BTH) and major adverse events (MAVEs)
ADDITIONAL END POINTS
Patients on FABHALTA experienced an annualized rate of clinical breakthrough hemolysis of 0.07 and 0.67 with C5is3,*,✝
The data from this additional analysis are descriptive in nature, presented for observation only. No formal conclusions or comparisons between the 2 treatment arms can be made. The planned power for the hypothesis testing was low.
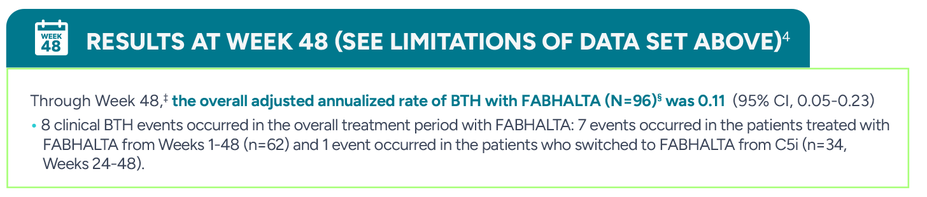
During the 24-week randomized treatment period, 2/62 patients (0.07 annualized rate) experienced clinical BTH with FABHALTA vs 6/35 (0.67 annualized rate) with C5is (eculizumab or ravulizumab) (95% CI, 0.02-0.61)
*Clinical BTH was defined as meeting clinical criteria (either decrease of Hb level ≥2 g/dL compared with the last assessment or within 15 days; or signs or symptoms of gross hemoglobinuria, painful crisis, dysphagia, or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared with the last 2 assessments).3
†Assessed between Days 1 and 168.3
‡BTH and MAVEs were reported from the start of FABHALTA to Day 336.4
§Patients who continued FABHALTA in the 24-week extension period: n=62; patients who switched from anti-C5 antibody to FABHALTA in the 24-week extension period: n=34.4
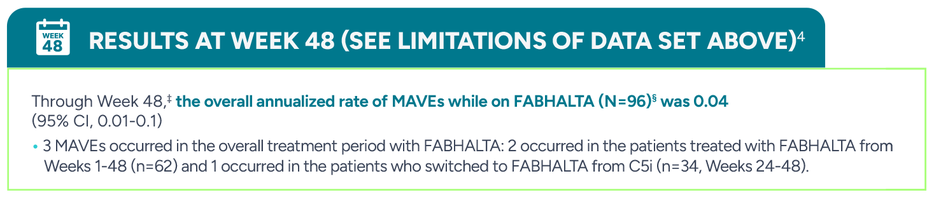
There was no significant statistical difference in the annualized rates of major adverse vascular events (MAVEs)|| between FABHALTA and C5is (0.03 vs 0, respectively; 95% CI, -0.03-0.10)3,†
The data from this additional analysis are descriptive in nature, presented for observation only. No formal conclusions or comparisons between the 2 treatment arms can be made.
During the 24-week randomized treatment period, 1 patient experienced transient ischemic attack in the FABHALTA arm, which was deemed unrelated to treatment by the investigator, vs 0/35 with C5is (eculizumab or ravulizumab)
†Assessed between Days 1 and 168.3
||The definition of MAVEs included: acute peripheral vascular occlusion, amputation, cerebrovascular accident, dermal thrombosis, gangrene, hepatic/portal vein thrombosis, mesenteric/visceral arterial thrombosis, myocardial infarction, pulmonary embolus, renal arterial or vein thrombosis, thrombophlebitis/deep vein thrombosis, transient ischemic attack, and unstable angina.3
‡BTH and MAVEs were reported from the start of FABHALTA to Day 336.4
§Patients who continued FABHALTA in the 24-week extension period: n=62; patients who switched from anti-C5 antibody to FABHALTA in the 24-week extension period: n=34.4
APPOINT: A study of complement inhibitor–naive adults with PNH1
Safety profile of FABHALTA in the APPOINT trial
Adverse reactions reported in >5% of adults with PNH treated with FABHALTA in APPOINT (24-week core treatment period)1
ADVERSE REACTIONS | FABHALTA (N=40) n (%) |
Headachea | 11 (28) |
Viral infectionb | 7 (18) |
Nasopharyngitisc | 6 (15) |
Rashd | 4 (10) |
Abdominal paina | 3 (8) |
Diarrhea | 3 (8) |
Lipid disordere | 3 (8) |
aIncludes similar terms.
bViral infection contains: COVID-19, herpes zoster, oral herpes, nasal herpes, influenza A virus test positive, influenza.
cNasopharyngitis contains: rhinitis allergic, upper respiratory tract infection, pharyngitis, rhinitis.
dRash contains: dermatitis allergic, acne, erythema multiforme, rash maculopapular, rash erythematous.
eLipid disorder contains: dyslipidemia, blood cholesterol increased, low-density lipoprotein increased, hypercholesterolemia, blood triglycerides increased, hyperlipidemia.
Serious adverse reactions were reported in 2 (5%) patients with PNH who received FABHALTA. They included COVID-19 and bacterial pneumonia
Bacterial infectionf and nausea were reported in 2 patients each (5%). Dizziness and urticaria were reported in 1 patient each (3%)
fBacterial infection contains: pyelonephritis, urinary tract infection, bronchitis bacterial, bronchitis haemophilus, cholecystitis, folliculitis, cellulitis, arthritis bacterial, sepsis, klebsiella infection, staphylococcal infection, Pseudomonas infection, hordeolum, pneumonia bacterial.
No patient discontinued FABHALTA due to an adverse reaction in the 24-week core treatment period or during the 24-week extension phase of the APPOINT trial5
Clinical breakthrough hemolysis and MAVEs
ADDITIONAL END POINTS
The data from this additional analysis are exploratory; therefore, not subject to family-wise Type 1 error control, and presented for observation only. No formal conclusions can be made.
During the 24-week core treatment period, no clinical BTH¶ or MAVEs# were observed in patients on FABHALTA5
¶Clinical BTH was defined as meeting clinical criteria (either decrease of Hb level ≥2 g/dL compared with the latest assessment or within 15 days; or signs or symptoms of gross hemoglobinuria, painful crisis, dysphagia, or any other significant clinical PNH-related signs and symptoms) and laboratory criteria (LDH >1.5 x ULN and increased as compared with the last 2 assessments).5
#The definition of MAVEs included: acute peripheral vascular occlusion, amputation, cerebrovascular accident, dermal thrombosis, gangrene, hepatic/portal vein thrombosis, mesenteric/visceral arterial or venous thrombosis or infarction, myocardial infarction, pulmonary embolus, renal arterial or vein thrombosis, thrombophlebitis/deep vein thrombosis, transient ischemic attack, and unstable angina.5
**BTH and MAVEs were reported from the start of FABHALTA to Day 336.4
Rollover extension program: 2-year long-term safety data in adults from APPLY and APPOINT
Safety profile of FABHALTA through 2 years
Most frequent AEs reported in ≥10% of patients7
Click on image to enlarge.
††The 'n' values reflect the number of patients with at least 1 event.7
‡‡The occurrence rate (number of episodes per 100 patient-years) was calculated as 100 times (the total number of AE episodes from all patients in the population divided by the total number of patient-years).7
§§Total exposure in patient-years is computed as (sum of the duration of on treatment periods over patients, in days) divided by 365.25.7
For TEAEs through 2 years, a frequency threshold of 10% or higher was selected. This is to account for the expected increase in frequency of TEAEs that is due to the longer observation period (2 years of exposure to FABHALTA in the REP; 24 weeks exposure in APPLY and APPOINT).7
8 patients discontinued FABHALTA in the APPLY and APPOINT 48-week studies as well as the 2-year REP: 2 patients in the 48-week studies (pregnancy), 4 patients during the REP (2 deaths, 1 adverse event, 1 physician decision), and 2 patients did not enter the REP program at all (eligibility, delayed IRB approval).7,8
Clinical breakthrough hemolysis and major adverse vascular events through 2 years
ADDITIONAL END POINTS
The data are for observation only. No formal conclusions can be made.
Click on image to enlarge.
BTH:
Adult patients with PNH on FABHALTA experienced 8.3 clinical BTH events per 100 PY (22 events, 95% CI 4.4-15.9)7
MAVEs:
Adult patients with PNH on FABHALTA experienced 1.5 MAVEs*** per 100 PY (4 events, 95% CI 0.5-5.0)9
||The 'n' values reflect the number of patients with at least 1 event.9
¶¶The occurrence rate (number of episodes per 100 patient-years) was calculated as 100 times (the total number of AE episodes from all patients in the population divided by the total number of patient-years).9
##Total exposure in patient-years is computed as (sum of the duration of on treatment periods over patients, in days) divided by 365.25.9
***The definition of MAVEs included: acute peripheral vascular occlusion, amputation, cerebrovascular accident, cerebral venous occlusion, dermal thrombosis, gangrene, hepatic/portal vein thrombosis, mesenteric/visceral arterial or vein thrombosis or infarction, myocardial infarction, pulmonary embolus, renal arterial or vein thrombosis, thrombophlebitis/deep vein thrombosis, transient ischemic attack, and unstable angina.10
A study of adults with PNH with Hb ≥10 g/dL who switched from a stable C5i regimen (eculizumab or ravulizumab) to FABHALTA10
Safety profile of FABHALTA in the APPULSE trial
Adverse reactions reported in >5% of adults with PNH treated with FABHALTA in APPULSE (24-week treatment period)11
ADVERSE REACTIONS | FABHALTA |
Headache | 17.3% |
Nasopharyngitis | 17.3% |
Viral infection | 13.5% |
Diarrhea | 11.5% |
Nausea | 11.5% |
Bacterial infection | 9.6% |
Lipid disorder | 7.7% |
Thrombocytopenia | 5.8% |
A serious adverse reaction (bacterial pneumonia) was reported in 1 patient (2%).
ADDITIONAL END POINTS
The data are for observation only. No formal conclusions can be made.
During the 24-week treatment period, no clinical BTH††† or MAVEs‡‡‡ were observed in patients on FABHALTA10
One patient discontinued FABHALTA due to an adverse reaction (palpitations)11
†††Rate of occurrence through Day 168. Summary measure was occurrences per year.10
‡‡‡The definition of MAVEs included: acute peripheral vascular occlusion, amputation, cerebrovascular accident, cerebral venous occlusion, dermal thrombosis, gangrene, hepatic/portal vein thrombosis, mesenteric/visceral arterial or vein thrombosis or infarction, myocardial infarction, pulmonary embolus, renal arterial or vein thrombosis, thrombophlebitis/deep vein thrombosis, transient ischemic attack, and unstable angina.10