FABHALTA was studied in both C5i-experienced (eculizumab or ravulizumab) and complement inhibitor–naive adults with PNH
Explore data to guide your treatment approach for patients with PNH who are ready to start or switch their treatment1-3
2-year rollover extension program
Patients from APPLY and APPOINT who entered the rollover extension program (REP)3,4
The APPLY study was a head-to-head trial of FABHALTA vs C5is (eculizumab or ravulizumab) in C5i-experienced adults with PNH and Hb <10 g/dL1
Phase 3, open-label, active comparator–controlled study1,5,6
Click on image to enlarge.
*Randomization was stratified based on prior C5i treatment and transfusion history within the last 6 months.1
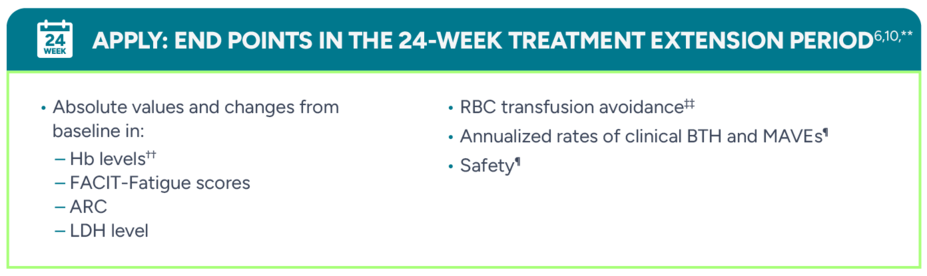
PRIMARY END POINTS: RANDOMIZED TREATMENT PERIOD1
Proportion of patients achieving sustained Hb increase of ≥2 g/dL† from baseline without a need for RBC transfusions‡ after 24 weeks
Proportion of patients achieving sustained Hb level of ≥12 g/dL† without a need for RBC transfusions after 24 weeks‡
ADDITIONAL END POINTS: RANDOMIZED TREATMENT PERIOD1,5
RBC transfusion avoidance§
Change from baseline† in:
- Hb levels (g/dL)‖
- FACIT-Fatigue scores
- Absolute reticulocyte count (ARC) (109/L)
- Lactate dehydrogenase (LDH) levelOccurrence of major adverse vascular events (MAVEs)¶
Occurrence of clinical breakthrough hemolysis (BTH)¶,#
Safety¶
In the APPLY study analysis:
All end points were based on central laboratory data6,7
95% CIs for end points in the randomized treatment period were based on the Sato variance estimator7
95% CIs in the extension period were based on the Clopper-Pearson method6
†Assessed between Days 126 and 168.1
‡Assessed between Days 14 and 168. Requiring RBCs refers to any patient receiving transfusions or meeting protocol-defined criteria.5,8
§Transfusion avoidance is defined as absence of administration of packed-RBC transfusions between Days 14 and 168.1,7
||Excludes values within 30 days post-transfusion in the randomized period.1
¶Throughout the study.5,6,9
#As per the protocol definition.5,8
¶Throughout the study.5,6,9
**No multiple imputation framework used for the analyses.6
††Excluding values within 30 days of RBC transfusion.6
‡‡Transfusion avoidance defined as patients who did not receive any transfusions between Days 14 and 336. Analysis considered patients who received transfusion between Days 14 and 336 after the start of FABHALTA treatment and with missing Day 336 assessment as nonresponders.6
Select baseline characteristics1,5:
(Baseline disease characteristics were generally well balanced between treatment groups.)
Group | FABHALTA | C5is |
Mean (SD) age (years) | 51.7 (16.9) | 49.8 (16.7) |
Mean (SD) Hb (g/dL) | 8.9 (0.7) | 8.9 (0.9) |
Mean (SD) LDH (U/L) | 269 (70) | 273 (85) |
Mean (SD) ARC (x 109/L) | 193 (84) | 191 (81) |
Mean (SD) disease duration | 11.9 (9.8) | 13.5 (10.9) |
Required ≥1 transfusion in | 56.5% | 60.0% |
Mean time on prior C5i | 3.8 | 4.2 |
Prior C5i treatment on | 64.5% | 65.7% |
Prior C5i treatment on | 35.5% | 34.3% |
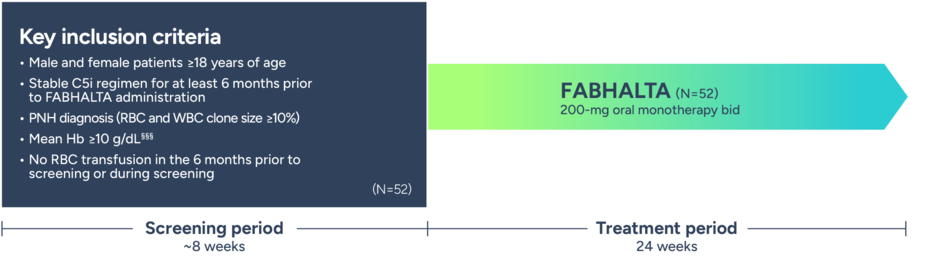
The APPOINT study was designed to evaluate the efficacy and safety of an oral monotherapy for complement inhibitor–naive patients with PNH1
Phase 3, single-arm, open-label, uncontrolled study1,6,8
Click on image to enlarge.
§§Confirmed by 2 measurements 2 to 8 weeks apart for patients not receiving an RBC transfusion during screening, or by 1 measurement during the first screening visit for patients receiving an RBC transfusion.8
||||Confirmed by at least 2 measurements 2 to 8 weeks apart during the screening period.8
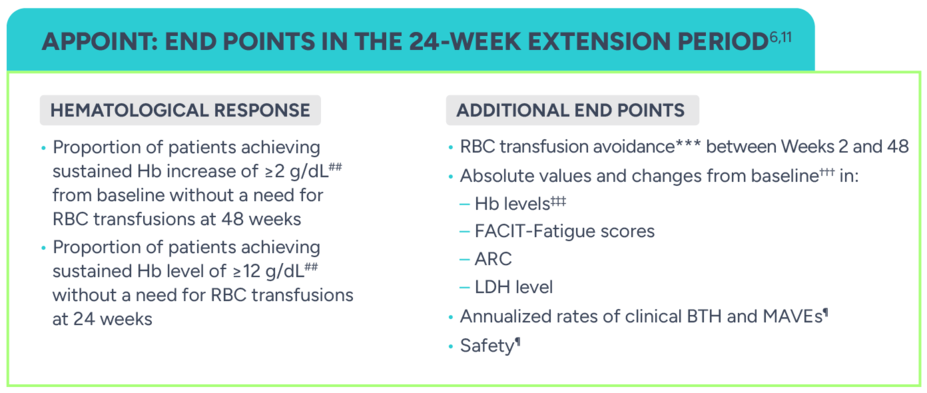
PRIMARY END POINT: CORE TREATMENT PERIOD1
Proportion of patients achieving sustained Hb increase of ≥2 g/dL† from baseline without a need for RBC transfusions‡ after 24 weeks
ADDITIONAL END POINTS: CORE TREATMENT PERIOD8
Proportion of patients with sustained Hb level of ≥12 g/dL† without a need for RBC transfusions‡
RBC transfusion avoidance¶¶
Change from baseline† in:
- Hb levels (g/dL)‖
- FACIT-Fatigue scores
- ARC (109/L)
- LDH levelOccurrence of MAVEs¶
Occurrence of clinical BTH¶,#
Safety¶
In the APPOINT study analysis:
All additional end points were exploratory1,7
Unless otherwise noted, all end points were based on central lab data1,7
95% CIs were based on the Clopper-Pearson method6,7
†Assessed between Days 126 and 168.1
‡Assessed between Days 14 and 168. Requiring RBCs refers to any patient receiving transfusions or meeting protocol-defined criteria.5,8
||Excludes values within 30 days post-transfusion in the randomized period.1
¶Throughout the study.5,6,9
#As per the protocol definition.5,8
¶¶Transfusion avoidance is defined as absence of administration of packed-RBC transfusions between Days 14 and end of study period.1,7
¶Throughout the study.5,6,9
##Based on observed and central laboratory data assessed at a single Week 36 (Day 336) visit. Patients who received transfusions between Days 14 and 336 or were missing a Day 336 assessment were considered nonresponders.6
***Based on observed data. Analysis considered patients who received transfusion between Days 14 and 336 as failures.6
†††Adjusted mean assessed at a single Week 48 visit.6
‡‡‡Hb data during the core treatment period and within 30 days post-transfusion were considered missing.6
Select baseline characteristics1,8:
Group | FABHALTA |
Mean (SD) age (years) | 42.1 (15.9) |
Mean (SD) Hb (g/dL) | 8.2 (1.1) |
Mean (SD) LDH (U/L) | 1699 (683) |
Mean (SD) ARC (x 109/L) | 154 (64) |
Mean (SD) disease duration (years) | 4.7 (5.5) |
Required ≥1 transfusion in last 6 months | ~70 |
The long-term efficacy and safety of FABHALTA were evaluated through 2 years in patients from APPLY and APPOINT who entered the rollover extension program (REP)14
The REP is an ongoing open-label, single-arm, multicenter, phase 3b rollover extension study to assess the long-term safety, tolerability, and efficacy of iptacopan 200 mg taken orally twice daily in adults with PNH who completed the treatment extension periods of a Novartis-sponsored phase 2 study or any phase 3 clinical study (without tapering), including the APPLY and APPOINT studies. The data presented is the 2-year long term APPLY and APPOINT safety and efficacy from REP.14,15
The following assessments were included3,4:
Hematological responses, defined as the percentage of patients with an increase of ≥2 g/dL in Hb level from baseline and percentage of patients achieving a Hb level of ≥12 g/dL, without RBC transfusion in the prior 12 months or irrespective of RBC transfusions received during the treatment period
Proportion of patients achieving transfusion avoidance
Changes from baseline in Hb levels, FACIT-Fatigue scores, and LDH levels
Occurrences of clinical BTH and MAVEs
Safety
The first day of iptacopan treatment in the parent studies is considered as the baseline.14
The APPULSE study was designed to evaluate a switch to FABHALTA in patients with Hb ≥10 g/dL who are ready to switch from their stable C5i regimen (eculizumab or ravulizumab)2
Phase 3, multicenter, single-arm, open-label study2
PRIMARY END POINT2
Change from baseline in Hb levels after switching from a C5i||||||
ADDITIONAL END POINTS
Proportion of patients with sustained Hb level of ≥12 g/dL¶¶¶ without a need for RBC transfusions###
RBC transfusion avoidance###
Change from baseline in:
FACIT-Fatigue scores****
ARC (109/L)††††
LDH levels‡‡‡‡
Occurrence of MAVEs§§§§
Occurrence of clinical BTH§§§§
Safety||||||||
All end points in the APPULSE study analysis were based on central laboratory data.
§§§Mean Hb ≥10 g/L over a period of 6 months before screening visit and confirmed by 2 different samples during the screening period.2
||||||Assessed as mean of visits between Days 126 and 168 compared with baseline, defined as mean of Hb collected at screening (2 samples) and Day 1.2
¶¶¶Assessed between Days 126 and 168 in the absence of RBC transfusions between Days 1 and 168, on 3 of 4 measurements taken at the visits occurring in the last 6 weeks. 95% CIs were based on the Clopper-Pearson method.2,3
###Transfusion avoidance is defined as absence of administration of packed-RBC transfusions between Days 1 and 168. 95% CIs were based on the Clopper-Pearson method.2
****Assessed at Days 84 and 168.2
††††Change from baseline as mean of visits between Days 126 and 168.2
‡‡‡‡Percentage change from baseline as mean of visits between Days 126 and 168.2
§§§§Rate of occurrence through Day 168. Summary measure was occurrences per year.2
||||||||Throughout the study.2
Select baseline characteristics2
Group | FABHALTA |
Mean (SD) age (years) | 46.0 (13.7) |
Mean (SD) Hb (g/dL) | 11.87 (1.3) |
Mean (SD) LDH (U/L) | 226.8 (69) |
Mean (SD) ARC (x 109/L) | 154.84 (76.2) |
Mean (SD) disease duration (years) | 10.80 (7.5) |
Mean time on prior C5i (years) | 3.5 |
Prior C5i treatment with eculizumab (% of patients) | 11.5% |
Prior C5i treatment with ravulizumab (% of patients) | 88.5% |